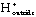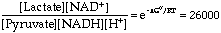
Glycogen
The brain and other tissues require a constant supply of blood glucose for survival. Glucose from the diet, though, arrives irregularly. Some tissues, particularly the liver and skeletal muscle, store glucose in a form that can be rapidly mobilized, glycogen. Liver glycogen is used to buffer the overall blood glucose level; glycogen is synthesized when blood glucose is high, and glycogen is degraded (with the resulting glucose released into the blood stream) when blood glucose is low, such as during the early stages of a fast. Muscle uses its glycogen stores for energy during strenuous exercise.
Structure of glycogen
Glycogen is a chain of glucose subunits held together by
a 1g4 glycosidic bonds, very much like starch (a-amylose). In contrast to starch, which is a single linear chain of glucose, glycogen is a branched structure. At the branch points, subunits are joined by a 1g6 glycosidic bonds. Branches occur every 8-10 residues.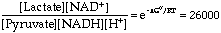
Glycogen synthesis
Glycogen is synthesized when blood glucose levels are high. This state is reflected inside liver cells by the presence of high levels of glucose-6-phosphate. G6P is first converted to G1P by phosphoglucomutase. This reaction is analogous to the reaction catalyzed by phosphoglycerate mutase in step 8 of glycolysis, and proceeds by a similar mechanism, with a bisphosphate intermediate.
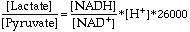
Conversion of G1P into glycogen is energetically unfavorable, so another source of energy input is required. This comes in the form of hydrolysis of UTP (uridine triphosphate). The high-energy phosphoanhydride bonds in UTP are equivalent to those in ATP. First, UTP is combined with G1P by UDP-glucose pyrophosphorylase.
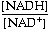
Since this reaction simply involves trading one phosphoanhydride bond for another,
DG is close to 0. However, the pyrophosphate is quickly hydrolyzed by inorganic pyrophosphatase into two molecules of inorganic phosphate, an essentially irreversible reaction. This coupling drives the synthesis of UDP-glucose in the forward direction.Next, glycogen synthase catalyzes the addition of this activated glucose subunit to the C4-hydroxyl group at the end of a glycogen chain (the non-reducing end).

UDP can be reconverted to UTP by nucleoside diphosphate kinase, which reversibly transfers
g-phosphate. Thus elongation of glycogen by one glucose requires energy input equivalent to hydrolysis of one molecule of ATP.UDP + ATP
D UTP + ADPGlycogen synthase can add a glucose subunit only onto a chain that is already at least 4 residues in length. De novo glycogen synthesis requires a primer, in the same way that DNA synthesis requires an RNA primer. Priming is performed by a specific protein, glycogenin, which autocatalyzes addition of an
a 1g4 linked glucose oligosaccharide chain to a specific tyrosine residue, using UDP-glucose as a substrate. After the chain is more than four residues long, glycogen synthase takes over. Glycogenin remains bound to the reducing end of glycogen (the C1 hydroxyl group at the right side of the pictures). Glycogen synthase works efficiently only when it is bound to glycogenin. Thus the number of glycogen granules in a cell is determined by the number of glycogenin molecules available, and the size of the granules is limited by the need for physical association between glycogenin and glycogen synthase. When the granule grows too large, the synthase stops working.Formation of branches is catalyzed by "branching enzyme", amylo (
a-1,4ga1,6) transglycosylase. This enzyme breaks off a chain of about 5 to 8 glucose residues from the growing end of glycogen by hydrolyzing an a 1g4 glycosidic linkage, and transfers the short chain to another residue in the same glycogen molecule that is at least four residues away from the cleavage point, forming an a 1g6 linkage.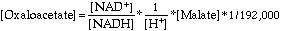
After the transfer, both the old C4 end (now at the end of the new branch) and the newly exposed C4 end (produced by the cleavage reaction) can be elongated by glycogen synthase. As soon as the new ends are long enough, they can again be branched. A mature glycogen granule may have seven layers of branches.
Branching gives glycogen two advantages over starch as a storage form of glucose. First, it is more soluble than its unbranched cousin. Second, the exposure of more C4 (nonreducing) ends means that glycogen can be both sythesized and degraded more quickly than a single starch chain with the same number of residues.
Glycogen degradation
When blood glucose is low, glycogen is reconverted to glucose in the liver. Glycogen phosporylase (sometimes called simply phosphorylase) catalyzes the cleavage of terminal glycogen residues by inorganic phosphate. Glycogen phosphorylase requires a covalently bound pyridoxal phosphate cofactor (vitamin B6).
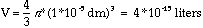
This cleavage reaction generates glucose-1-phosphate, which can be converted to G6P by phosphoglucomutase (see above). In muscle, this G6P is used directly for glycolysis. In liver, when blood glucose is low, G6P is hydrolyzed to glucose by glucose-6-phosphatase, and the resulting glucose is exported into the bloodstream for use in other tissues. Glucose-6-phosphatase is absent in muscle.
Recall that
a-amylase, secreted in saliva and in the small intestine, also cleaves a 1g4 glycosidic bonds, but it does so hydrolytically (using water to cleave) rather than phosphorolytically (using phosphate to cleave). If a-amylase were used for glycogen degradation, it would generate glucose rather than G1P. The glucose would have to be rephosphorylated in an ATP-requiring step before it could be used for glycolysis.Glycogen phosphorylase cannot cleave any terminal glucose residues that are fewer than four subunits away from the nearest branch point. It therefore cannot degrade glycogen completely, but leaves a "limit dextrin" with four residues in each branch. Complete degradation requires the action of a second, bifunctional enzyme, called "debranching enzyme."
The first activity of debranching enzyme, oligo (
a-1,4ga1,4) glucantransferase, transfers three of the four residues in the branch of a limit dextrin to the C4 end of another branch, leaving a single glucose attached at the branch point by the a 1g6 linkage. This reaction requires no energy, merely substituting one a 1g4 linkage for another. The resulting longer chain can again be degraded by glycogen phosphorylase, until only four residues remain to the nearest branch point.
The single glucose left attached by the
a 1g6 linkage is then hydrolyzed by the amylo-(a 1g6)-glucosidase activity, encoded by the same polypeptide chain as the glucantransferase. This reaction is a hydrolysis, not a phosphorolysis, so the product is glucose rather than G1P. Because of the need to hydrolyze the branched residues, recovery of phosphorylated glucose from glycogen is not 100% efficient.
Summary of glycogen metabolism

The key regulated enzymes in glycogen synthesis and degradation are glycogen synthase and glycogen phosphorylase, respectively. The action of both enzymes is regulated by the level of energy and metabolites available to the cell, as well as by circulating hormones including insulin, glucagon and epinephrine. They must be coordinately regulated, so that glycogen synthesis and degradation are not activated or inhibited simultaneously.
Glycogen synthase is allosterically activated by G6P, ensuring that glucose will be stored when it is abundant. Conversely, glycogen phosphorylase is inhibited by G6P, as well as by ATP and glucose. In muscle, glycogen phosphorylase is activated by AMP, which is present at high concentrations only under extreme conditions of anoxia and ATP depletion. Hormone-dependent regulation of glycogen synthase and phosphorylase is mediated by cyclic AMP (cAMP), a pathway we will discuss in more detail below.
Glycogen storage diseases
Defects in several of the enzymes involved in glycogen metabolism cause a family of inherited diseases.