Ongoing Research Topics
The overarching goal of the Herschlag Lab is to understand the fundamental behavior of RNA and proteins and, in turn, how these behaviors determine and impact biological catalysis and biology. The lab takes an interdisciplinary approach, spanning and integrating physics, chemistry and biology through fruitful interactions with collaborators and a wide range of techniques are employed.
Ketosteroid Isomerase Catalysis
 Understanding the mechanisms by which enzymes catalyze chemical reactions with enormous rate enhancements and exquisite specificity is central to biology. And knowledge of the chemical and physical basis of catalysis provides fundamental information that may facilitate the design of novel, artificial enzymes and aid the design of enzyme inhibitors that may act as drugs.
Understanding the mechanisms by which enzymes catalyze chemical reactions with enormous rate enhancements and exquisite specificity is central to biology. And knowledge of the chemical and physical basis of catalysis provides fundamental information that may facilitate the design of novel, artificial enzymes and aid the design of enzyme inhibitors that may act as drugs.
There have been astounding advances in the understanding of enzyme mechanism over the past decades. Nevertheless, fundamental questions remain, and indeed, much of the previous work has helped to bring these critical questions into focus.
Enzymes cannot be described as simply a collection of catalytic amino acids and cofactors. Rather, these groups come together with a precise active site arrangement in a unique, idiosyncratic environment created by the protein scaffold. It is the large number of interactions and this ‘context’ that distinguish enzymes from simple, small molecule catalysts and that must be explored to achieve the next level of understanding of enzymatic catalysis.
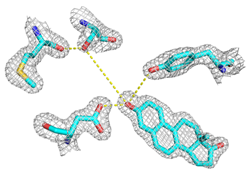
The bacterial enzyme ketosteroid isomerase (KSI) provides a remarkably tractable system for in-depth dissection of the mechanisms of catalysis used by enzymes: It catalyzes a simple, well-characterized chemical transformation; it has an established kinetic and thermodynamic reaction framework; it has available transition state analogs to allow structural and spectroscopic probes of active site interactions; it has known, high resolution x-ray crystal structures and is amenable to rapid determination of X-ray structures of new variants and complexes; it catalyzes equilibration of a single substrate/single product reaction, allowing enzyme/substrate complexes to be observed by crystallography and other structural techniques; it is amenable to a panoply of biophysical probes, including NMR and vibrational spectroscopy; and it can be generated semi-synthetically to allow the incorporation of series of unnatural amino acids to systematically and rationally test specific mechanistic proposals.
Some leading papers from the lab in the areas of enzyme mechanism and ketosteroid isomerase are:
See also: Faculty of 1000 Biology, 16 Oct. 2007 http://www.f1000biology.com/article/id/1091226/evaluation
See also: Nature 2008, 456, 45-47. http://www.nature.com/nature/journal/v456/n7218/full/456045a.html