
|
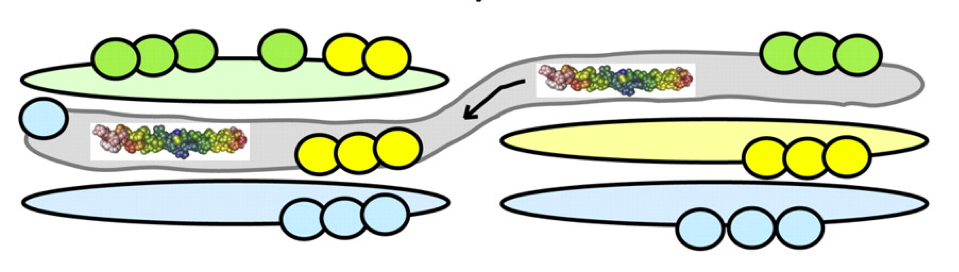
How the Golgi works: A cisternal progenitor model
Suzanne Pfeffer PNAS vol. 107, no. 46, 19614-19618 (2010)
The Golgi complex is a central processing component in the secretory pathway of eukaryotic cells. This essential component processes more than 30% of the proteins encoded by the human genome, yet we will do not fully understand how the Golgi is assembled and how proteins pass through it. Recent advances in our understanding of the molecular basis for protein transport through the Golgi and within the endocytic pathway provide clues to how this complex organelle may function and how proteins may be transported through it. Described here is a possible model for transport of cargo through a tightly stacked Golgi that involves continual fusion and fission of stable, "like" subcompartments and provides a mechanism to grow the Golgi complex from a stable progenitor, in an ordered manner.
READ FULL PUBLICATION
|
|
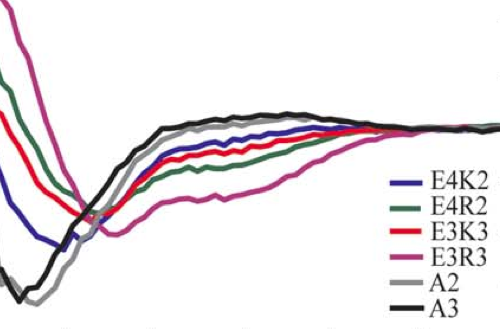
Helicity of short E-R/K peptides
Sommese et al. Protein Science vol. 19, is. 10, 2001-2005 (2010)
Understanding the secondary structure of peptides is important in protein folding, enzyme function, and peptide-based drug design. Previous studies of synthetic Ala-based peptides (>12 a.a.) have demonstrated the role for charged side chain interactions involving Glu/Lys or Glu/Arg spaced three (i, i+3) or four (i, i+4) residues apart. The secondary structure of short peptides (<9 a.a.), however, has not been investigated. In this study, teh effect of repetitive Glu/Lys or Glu/Arg side chain interactions, giving rise to E-R/K helices, on the helicity of short peptides was examined using circular dichorim. Short E-R/K-based peptides show significant helix content. Peptides containing one or more E-R interactions display greater helicity than those with similar E-K interactions. Significant helicity is achieved in Arg-based E-R/K peptides eight, six, and five amino acids long. In these short peptides, each additional i+3 and i+4 salt bridge has substantial contribution to fractional helix content. The E-R/K peptides exhibit a strongly linear melt curve indicative of noncooperative folding. The significant helicity of these short peptides with predictable dependence on number, position, and type of side chain interactions amkes them an important consideration in peptide design.
READ FULL PUBLICATION |
| |
Publications Archive
PubMed |

Stanford Biochemistry Department | Copyright © 2010 | All Rights Reserved






